Science and Services
Applying the science of quantitative approaches, from trial design to regulatory interactions. We support clients by reducing uncertainty in technical and regulatory processes, increasing confidence in the development of new medicines that can improve patients’ lives.
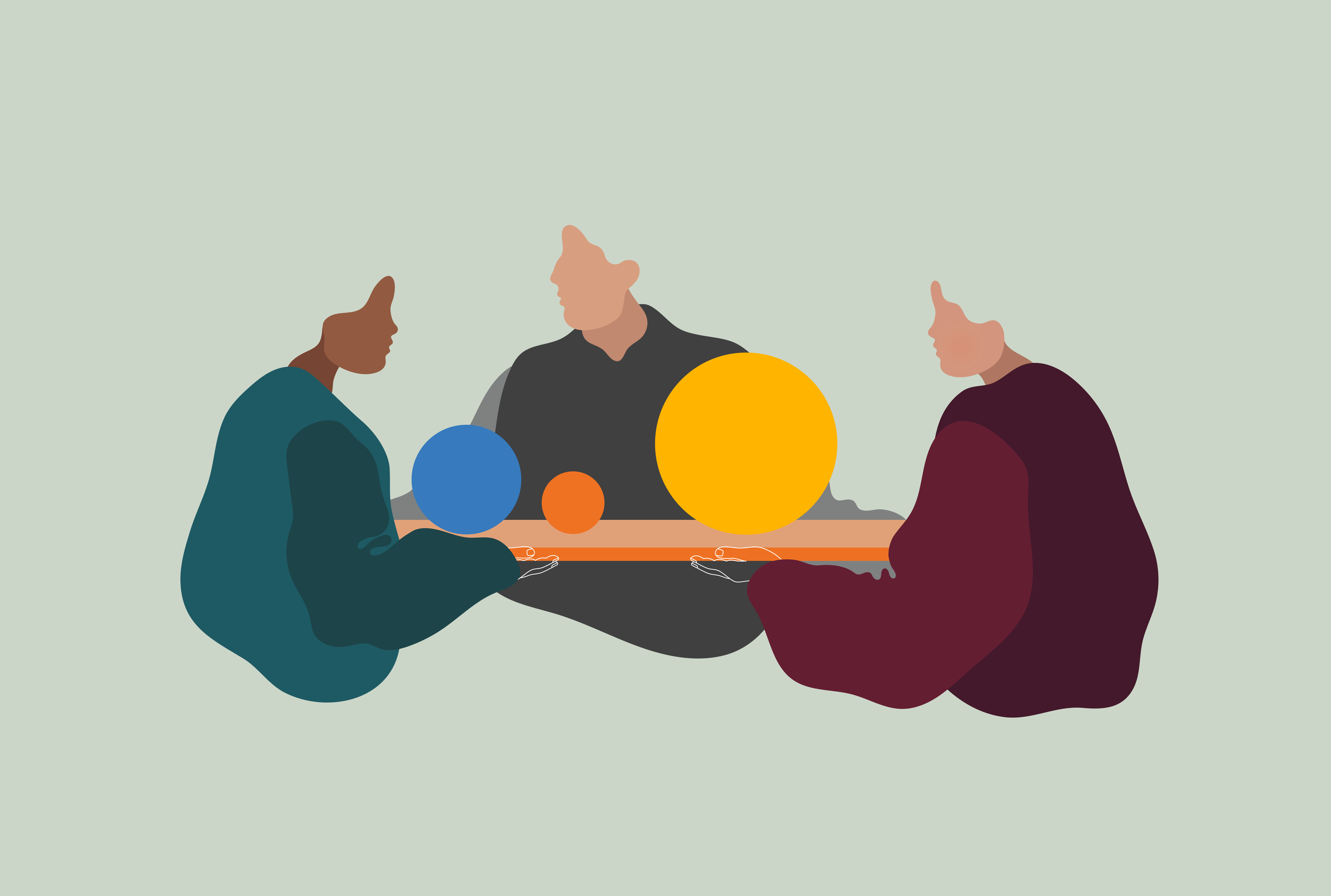
Pharmetheus specializes in quantitative approaches to drug research, development, and usage.
We offer regulatory-compliant solutions that ensure effective use of data allowing your team to more accurately characterize the potential drug effects and make confident decisions with sufficient precision.
To improve human health, we explore data, create models, and collaborate with our clients, colleagues, and the scientific community at large.
Explore Science & Services

Contexts of use
Development projects face numerous challenges, but with appropriate support, you can reduce risk and uncertainty along the process, aiding decision-making in a way of working adapted to the context of use.
Pharmetheus Platforms
Harness the power of data integration to support decision-making and achieve predictions beyond observations. Explore the different Pharmetheus Platforms we have established for this purpose.
Solutions
Strengthen your team from within, get access to essential knowledge and a broad skillset.
Case studies
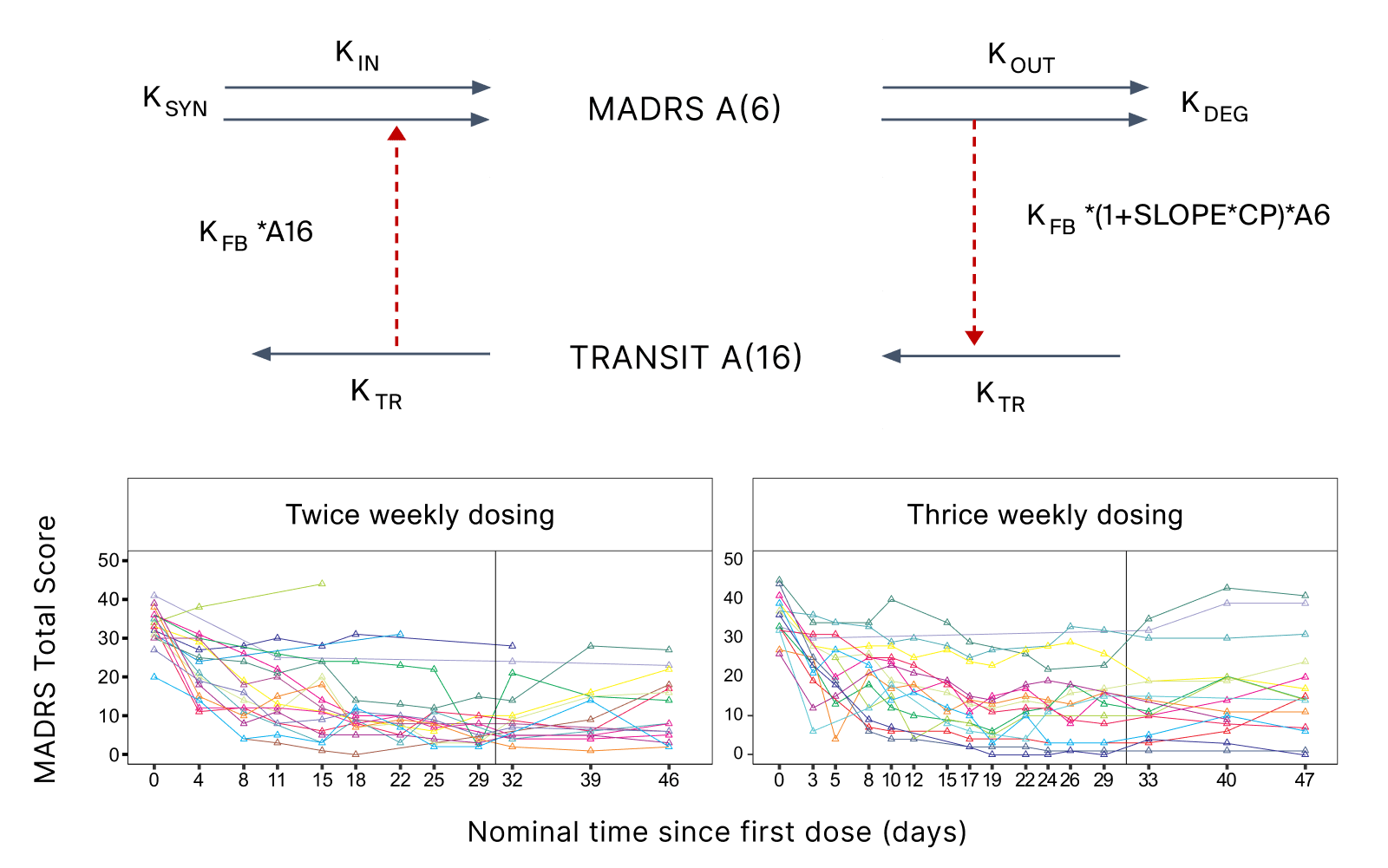
Assessment of the probability of a drug asset to exceed the target efficacy value in major depression disorder trials using literature data of ketamine and esketamine
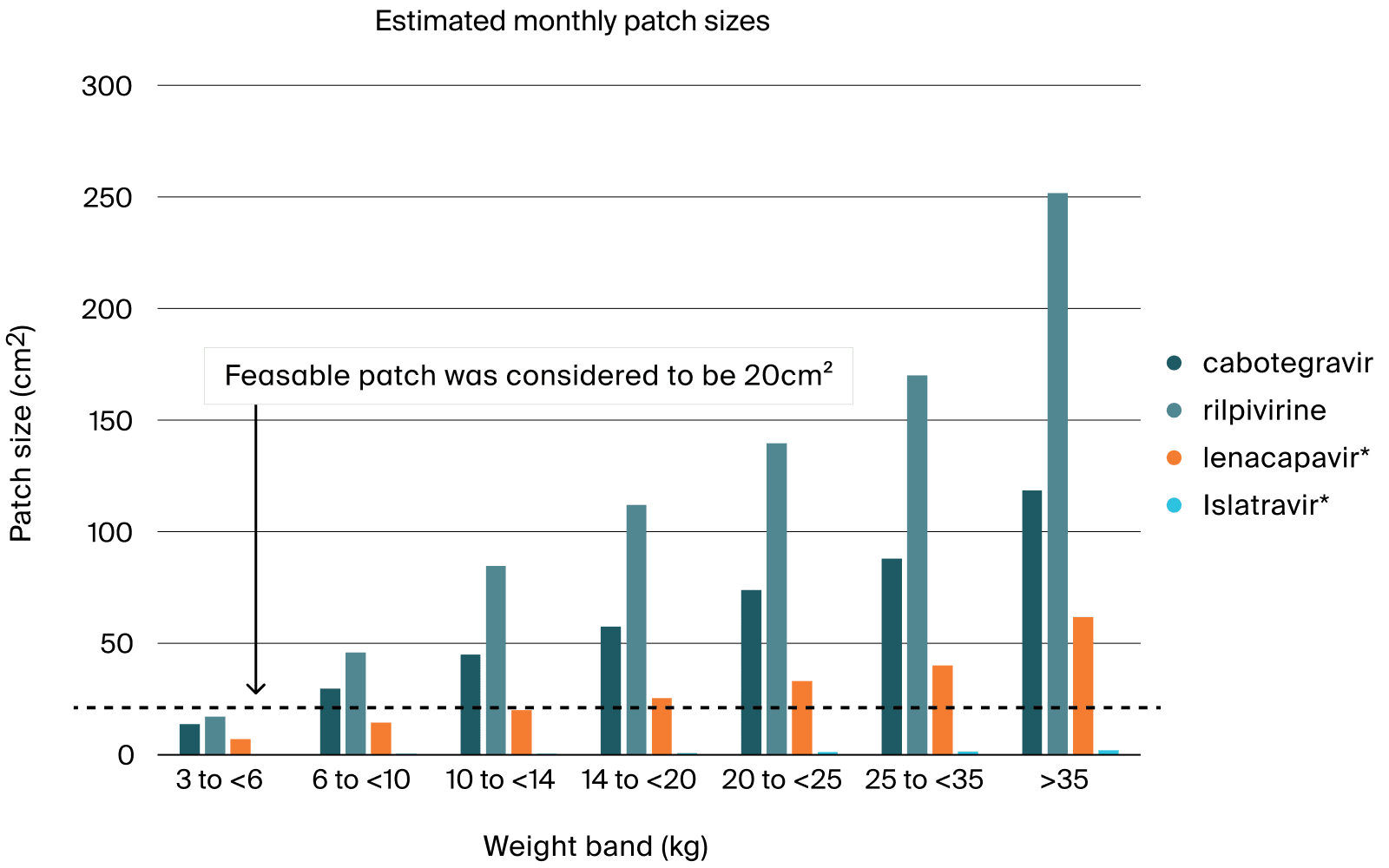
Model-informed microarray patches design for anti-retroviral drugs to treat children with HIV
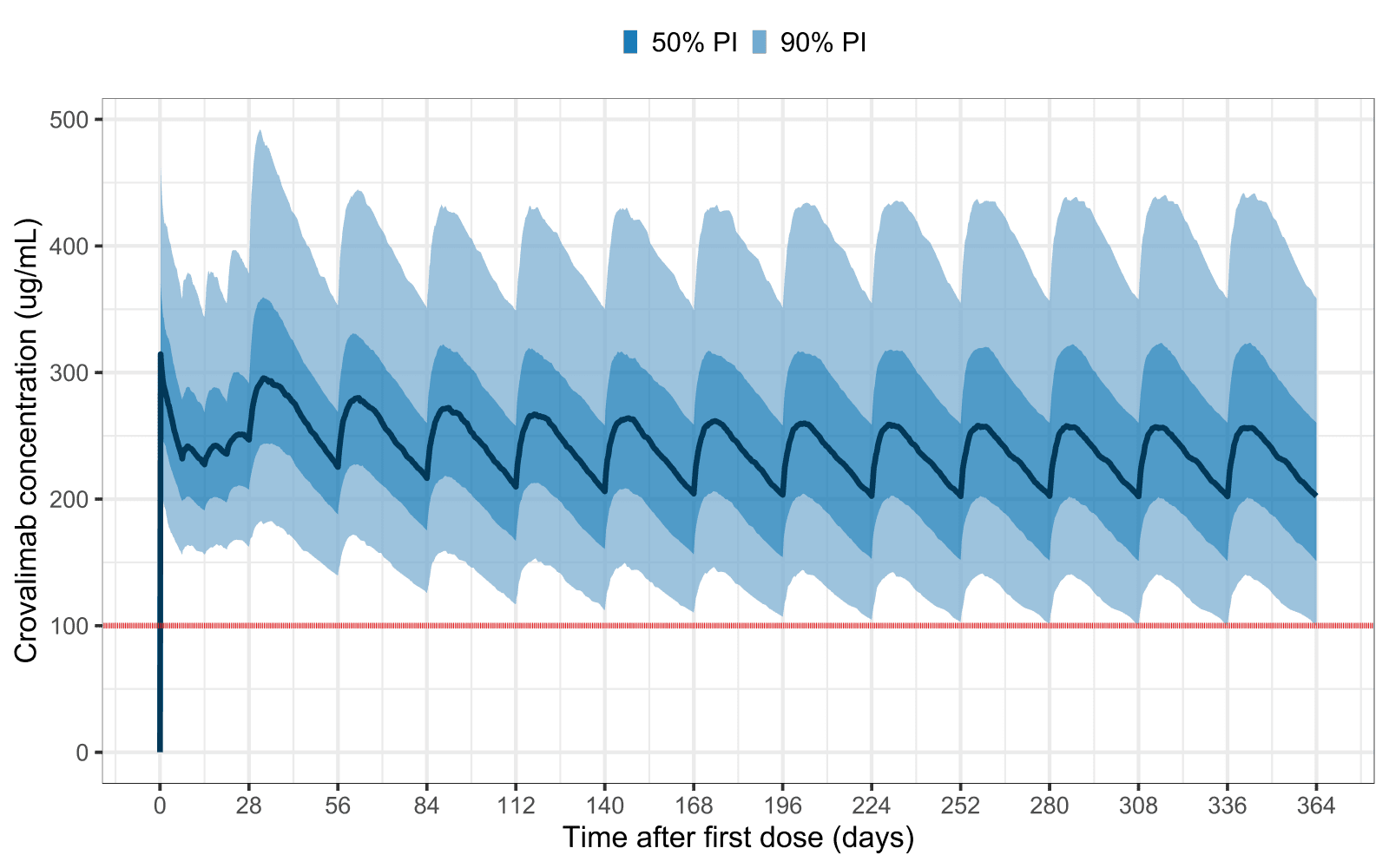
Crovalimab dose justification based on Phase 3 data in paroxysomal nocturnal hemoglobinurea patients
Academic collaborations
At Pharmetheus, we highly value collaborations with academic partners as part of our strategy of constant development and adding new, innovative, state-of-the-art solutions to our toolbox.
Publications
We help our clients access the necessary knowledge regarding a particular drug and disease, and drive better, model-informed, decisions and support them in putting the findings in words as we write the report for publication in peer-reviewed scientific journals and conference submissions. We also publish our own work and joint publications with partners and academic institutions.