About the case
- Therapeutic area: CNS
- Phase I-II
- Impact of Model Informed Drug development: Informing
Conclusion
Investments in clinical trials with low probability of success were avoided while dosing and designs of planned studies planned with the right assets were optimized (Figure 1).

Figure 1. Comparing the probability (% of simulated trial replicates) of exceeding the target efficacy value at the highest dose in a planned Phase II study
- Main challenges included the extrapolation of the single dose pharmacokinetics (PK) of the new assets in Phase I to the multiple dose scenarios of the planned Phase II study, as well as the assumption of similar efficacy of the new assets and ketamine or alternatively their comparison based on in vitro data.
- Pharmetheus supported this client with strategic advice and hands-on modeling and simulation to optimize the study design and facilitate the interpretation of observed study outcomes.
- Methodologically, this project involved literature review and data digitization. The digitized pharmacodynamic (PD) data (on the Montgomery-Åsberg Depression Rating Scale) were coupled with literature PK models for ketamine and esketamine using indirect response PKPD models with positive feedback. The proposed Phase II study design was simulated in multiple replicates. The two new assets were compared based on their PK properties and the corresponding exposure limits. The probability of exceeding various target values was calculated for different dose levels (Figure 2).
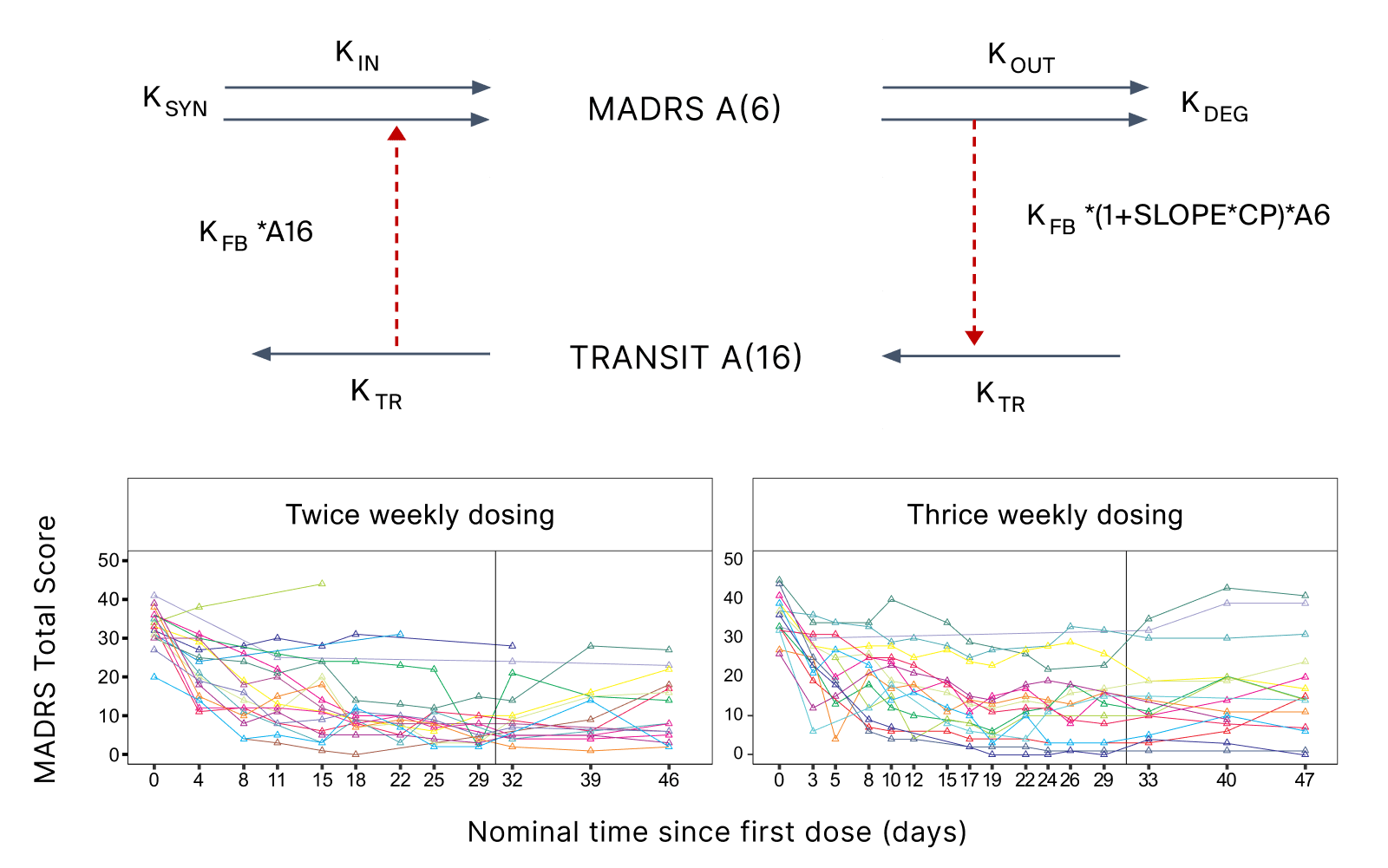
Figure 2. Overview of methodological approach