Regulatory interactions
Interactions such as consultations or Phase lll submission, end of Phase ll meetings, or others can provide great insights to the drug development team.
In handling regulatory interactions related to clinical drug development, there are several considerations to be made. With the right support, potential challenges can be mitigated.
Timely regulatory interactions
Whether preparing for the end of Phase II meetings (EOP2), filing after Phase III, or drafting a pediatric investigation/study plan (PIP/PSP), these interactions have a short turnaround time despite many stakeholders. Timely regulatory interactions, such as scientific consultations and regulatory matters, are key in the aforementioned matters. Other types of regulatory interactions include the FDA’s MIDD Pilot Program which facilitates integrating MIDD into drug applications and advancing its use.
Interactions support
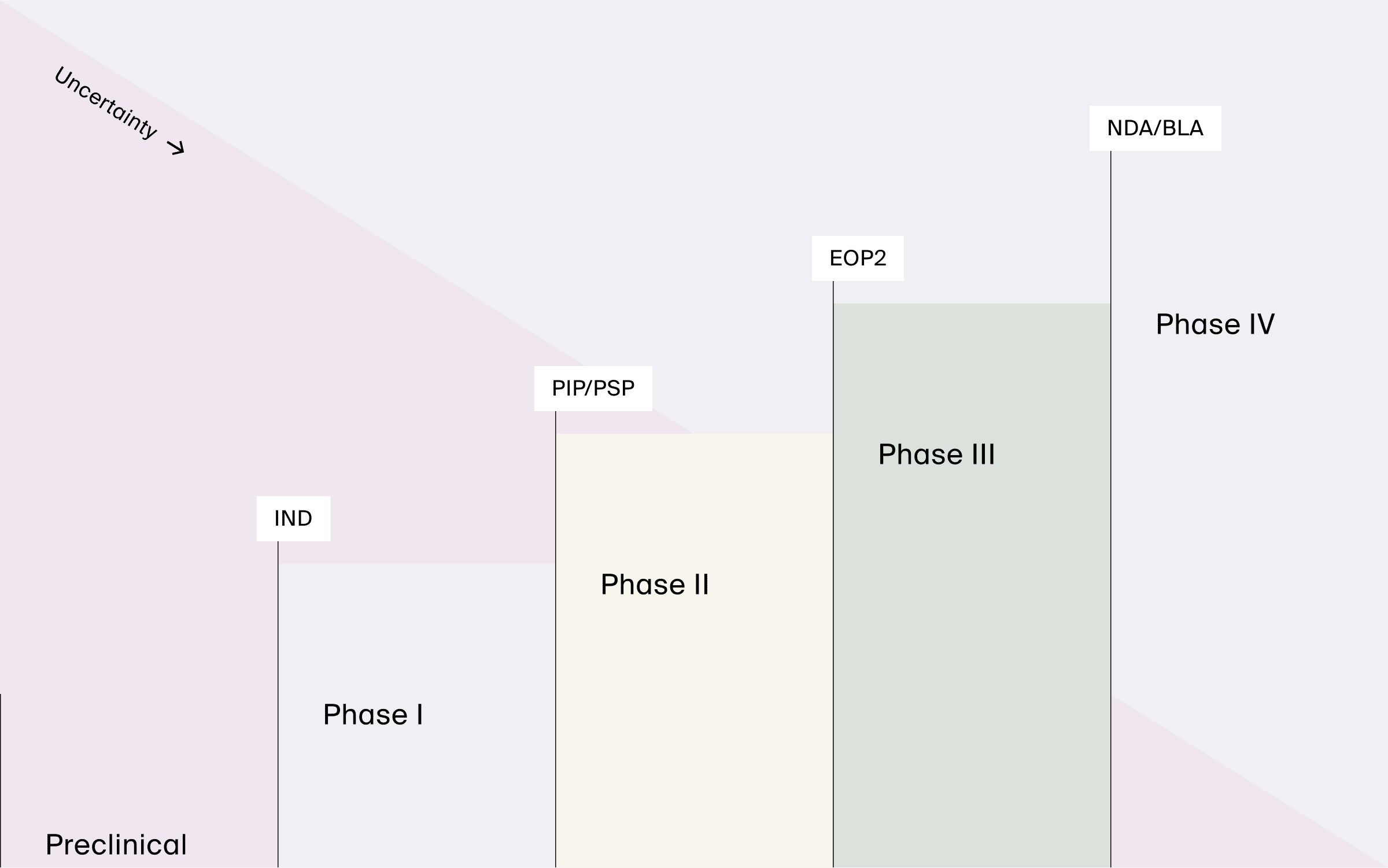
Our experience
- We have worked with FDA (US), EMA (EU), PMDA (Japan) and other national agencies, and have consultants with experience from the MPA (Sweden).
- We provide drug development support with comprehensive pharmacometrics analysis reports that serve your entire team and aid with timely regulatory interactions, such as scientific consultations and regulatory matters.
Recommended platforms
Recommended platforms in stages before and during regulatory interactions are:
To analyze both drug concentration (pharmacokinetic) and effects (pharmacodynamic) data using a population modeling approach. The adopted quantitative approach enables efficient integration of longitudinal information across subjects and across study protocols.
When the objective is to leverage accumulated knowledge to inform mechanistic analyses and or achieve predictions beyond observations.
A quantitative framework to support and inform development program strategies and decisions.
Recommended solutions
Pharmetheus is an experienced partner offering a reproducible reporting system that provides outsourced analysis, expert advice in the form of review or coaching, and embedded scientist project support. Learn more about the solutions we offer related to regulatory interactions.
At Pharmetheus, we understand the importance of providing efficient and effective support to our clients. Our milestone analysis solutions are designed to meet the unique needs of each project.
Pharmetheus offers a unique Embedded Scientist service, providing project support with essential knowledge in quantitative approaches.
Learn from our experts at Pharmetheus for review work, strategy advice, and coaching.