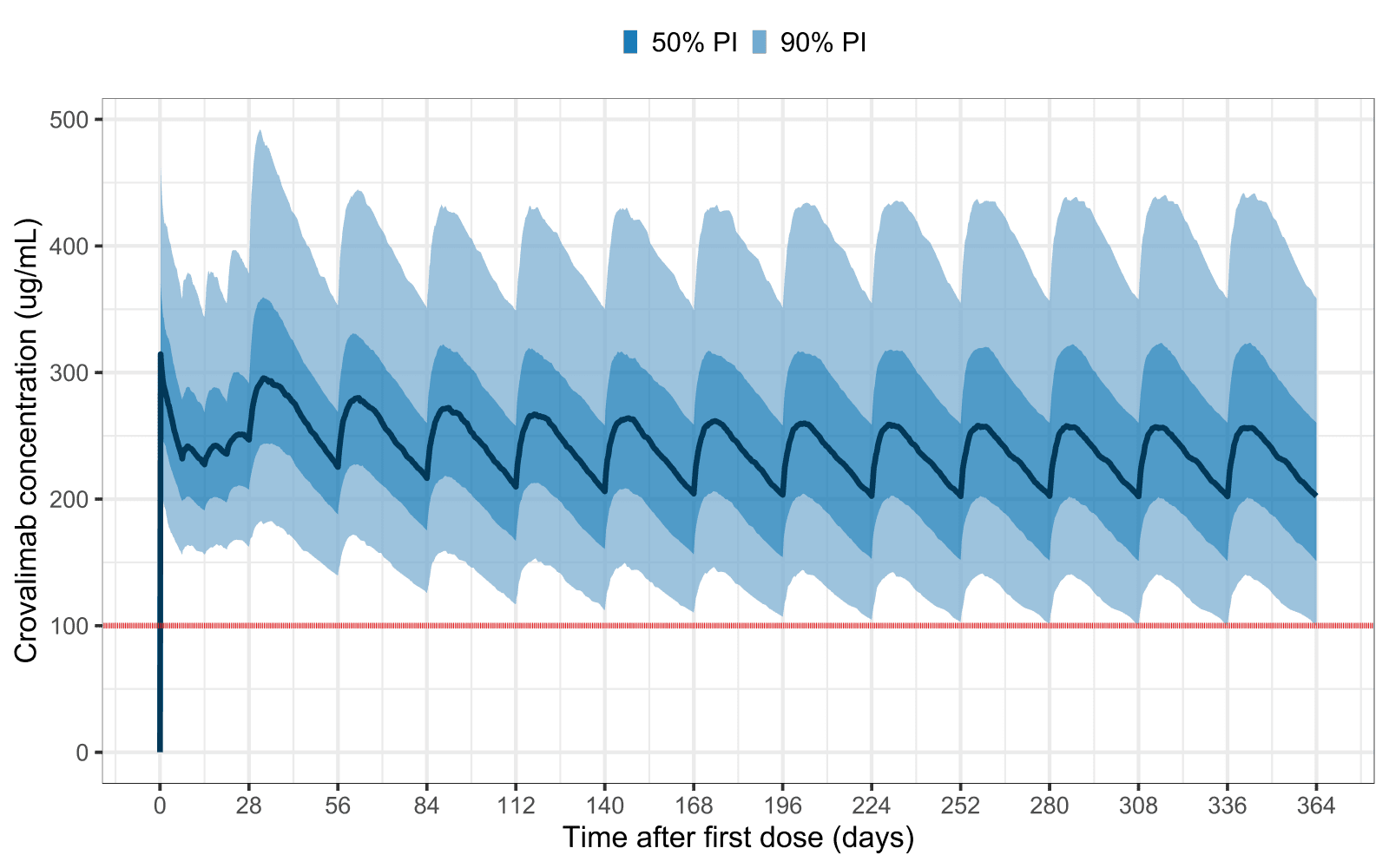
Pharmacokinetic characterization and exposure–response relationship of crovalimab in the COMMODORE 1, 2 and 3 and COMPOSER trials of patients with paroxysmal nocturnal haemoglobinuria
Background
Paroxysmal nocturnal haemoglobinuria (PNH) is an ultra-rare, clonal haematopoietic stem cell disorder that often leads to intravascular haemolysis, thrombosis, and a reduced quality of life.
This publication offers a detailed examination of crovalimab, a novel C5 inhibitor developed to address these challenges. Authored by Dr. Valérie Cosson, Dr. Andrea Henrich, and collaborators from Pharmetheus and F. Hoffmann-La Roche Ltd., the research integrates data from four clinical trials to investigate crovalimab’s pharmacokinetic profile under a weight-based, two-tiered dosing regimen, as well as its exposure–response relationship.
Key findings
The findings demonstrate that crovalimab achieves complete terminal complement activity inhibition, provides effective disease control, and maintains a safety profile comparable to existing C5 inhibitors. Notably, the study also highlights crovalimab’s potential to reduce treatment burden, offering a promising avenue for improving care in PNH.
Pharmetheus Affiliates
Connected case