About the case
- Therapeutic area: Infectious Diseases
- Development stage: Phase I
- Modeling strategy: PBPK-PBBM, MIDD (Model-Informed Drug Development)
- MIDD Impact category: Inform
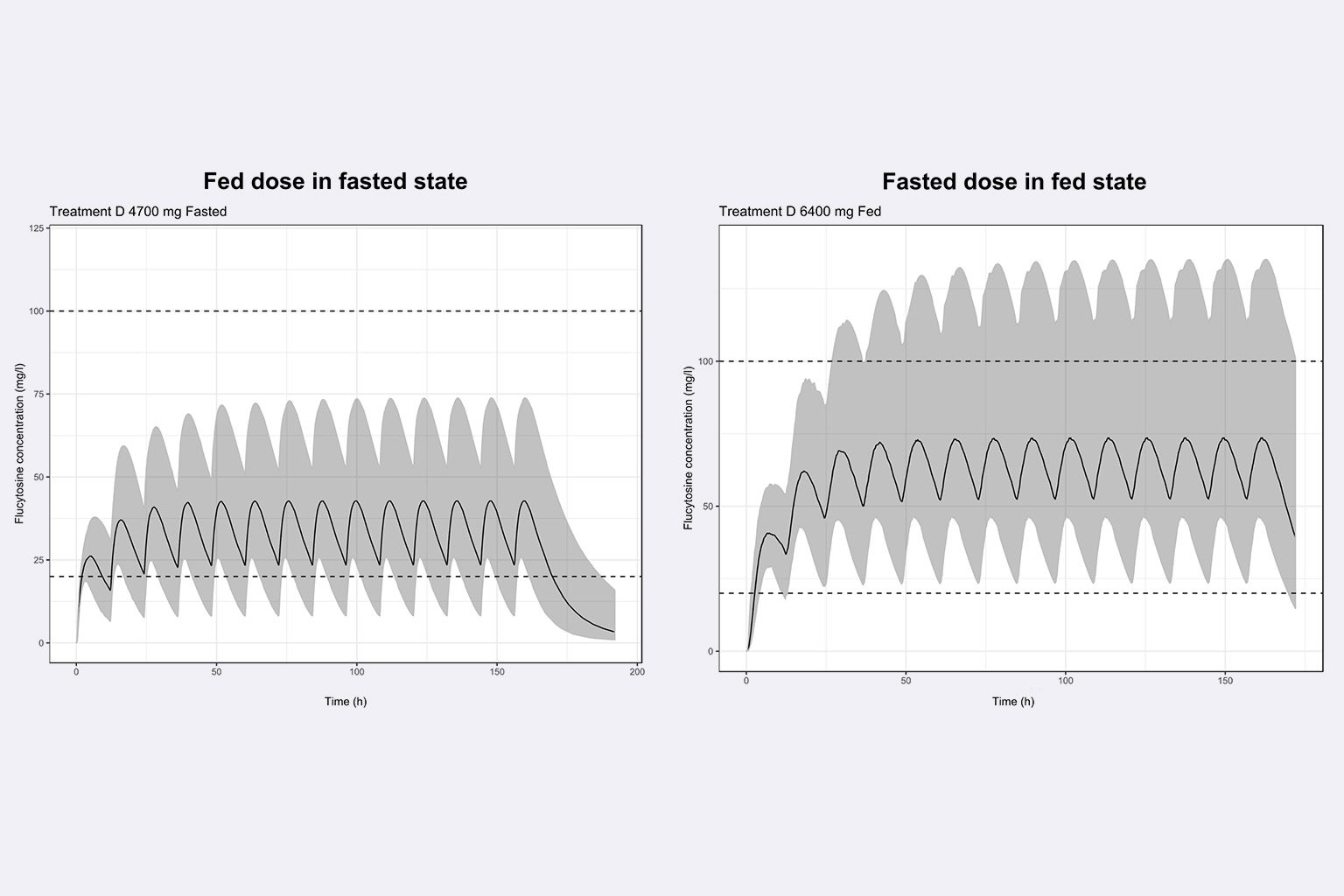
Using the previously developed PBPK model and based on assumptions about the food effect mechanism, PBPK predictions supported the design of the food interaction study to come. After the study completion, the model was refined confirming food effect mechanism hypotheses.
Principal question
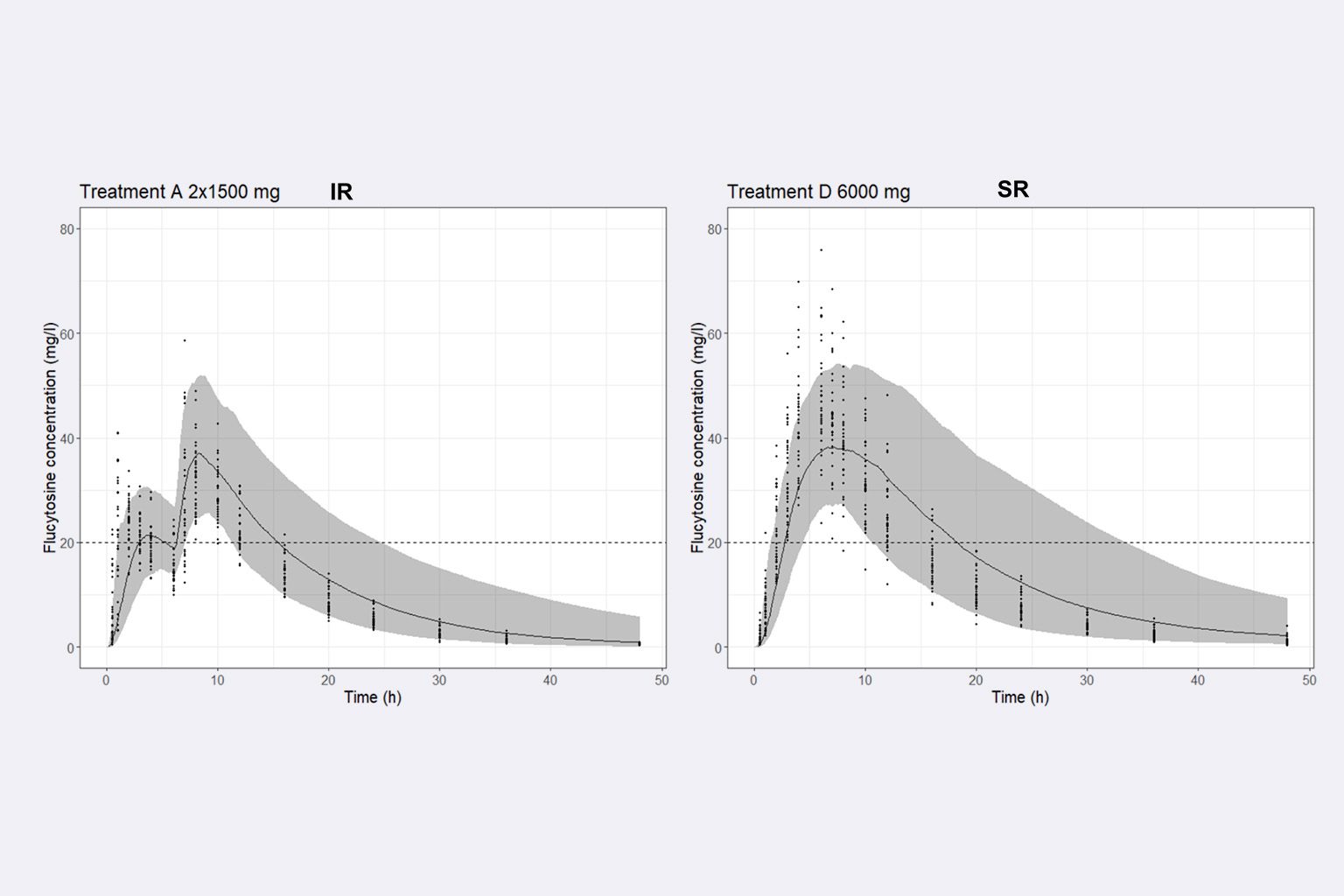
What is the food effect on flucytosine (5FC) PK profile after administration of a sustained release formulation in the context of a switch from immediate release to sustained release, for cryptococcal meningitis treatment in HIV patients?
Impact
This work supported the dose selection for a food interaction study with the SR formulation and later served to make recommendations toward food intake to patients (Medium impact).
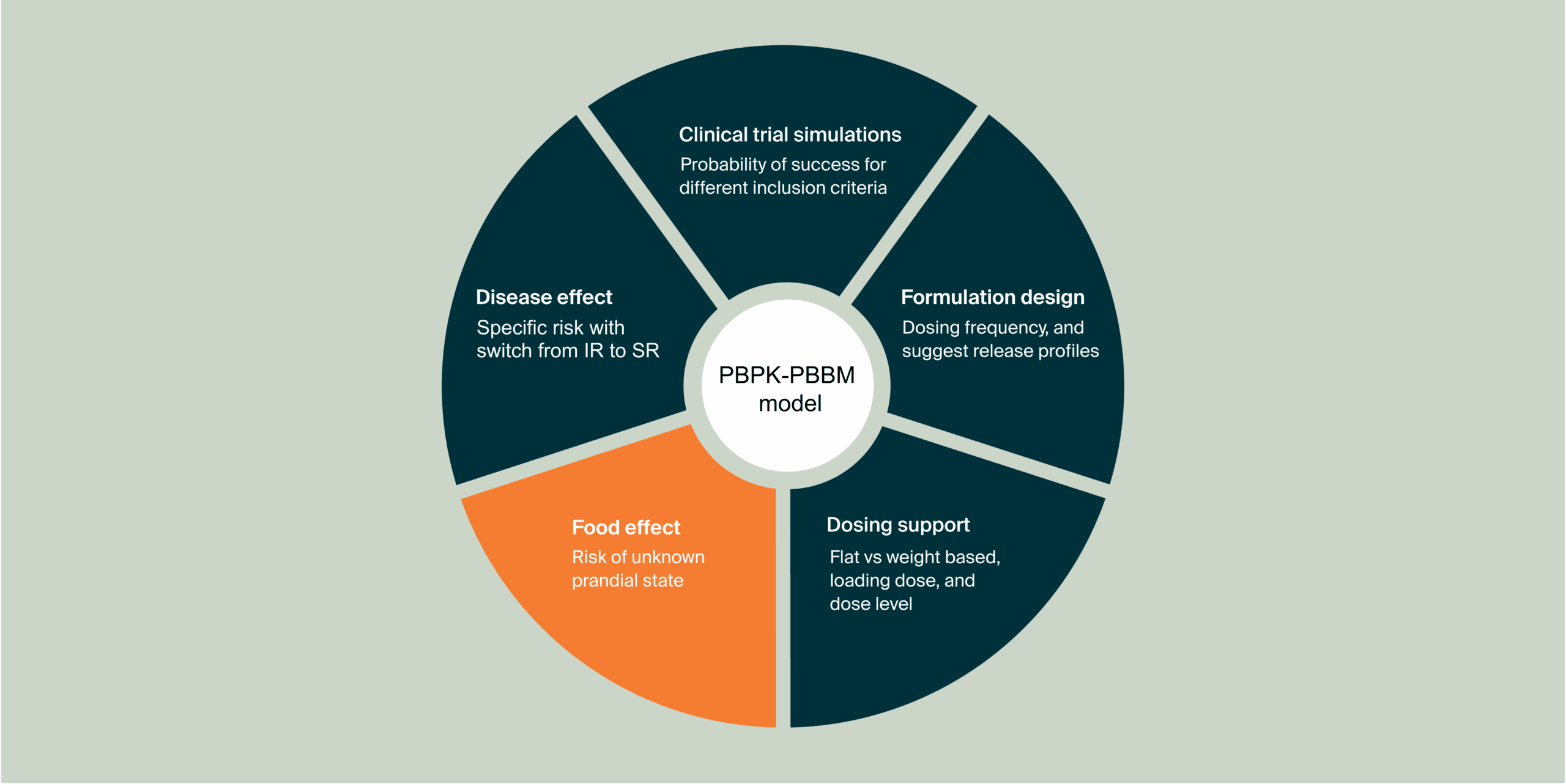
Main challenge(s)
- To predict the food effect on 5FC PK after administration of the sustained release formulation based on 5FC physicochemical properties and biological knowledge.
- To refine the legacy model using food interaction clinical data by testing hypotheses about the food interaction mechanism on 5FC release and absorption.
Pharmetheus role
Pharmetheus was responsible for the MIDD and the PBPK modelling and simulation, and participated in project team discussions about food interaction study design and food intake recommendation.
Methodological approach
Physiologically-based pharmacokinetics modeling (PBPK) of food effect implemented in PK-Sim® software.
See the next case in this series
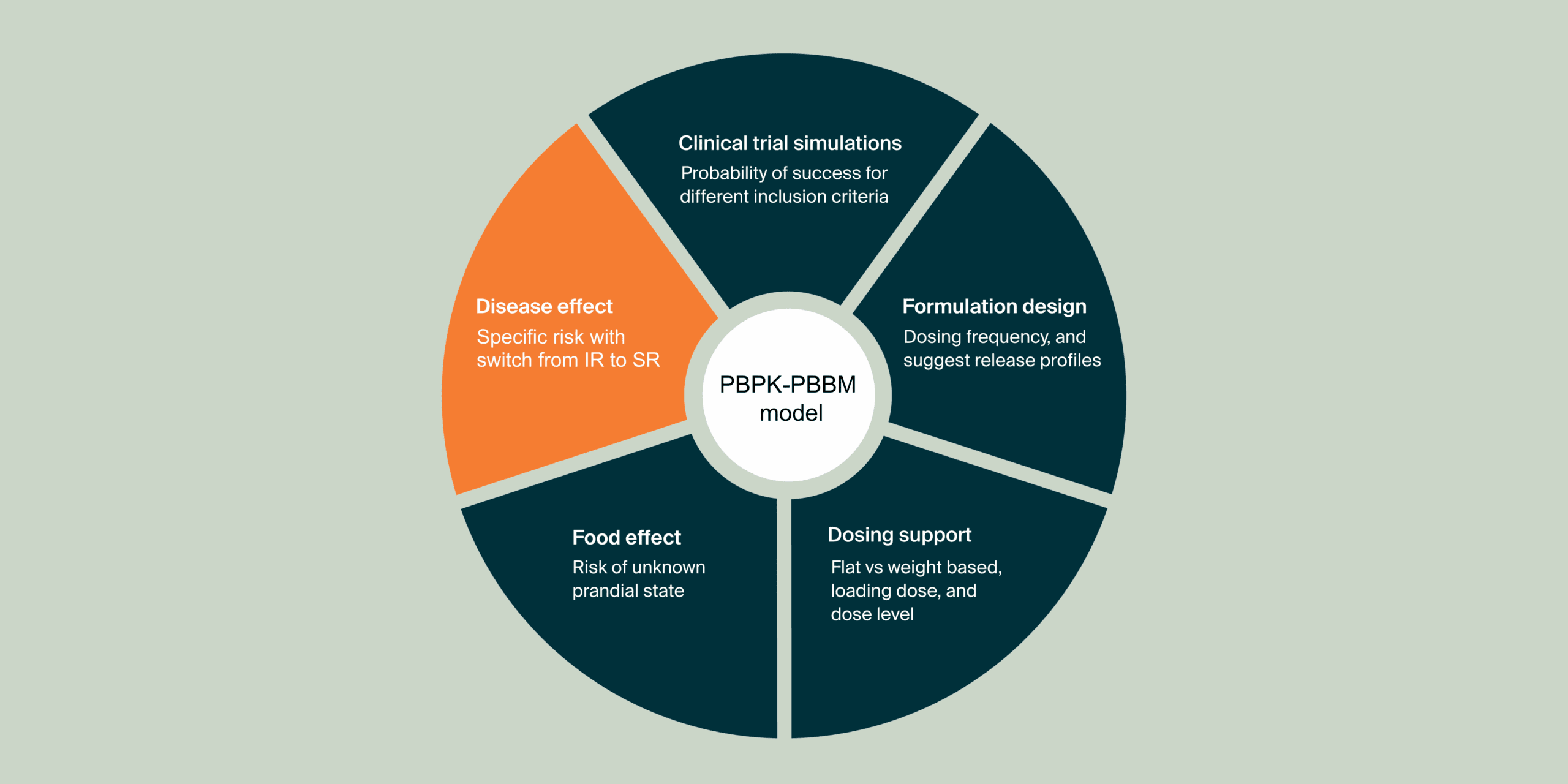
Disease effect prediction on PK in the context of a switch from an immediate release to a sustained release formulation.
In this PBPK-informed case study, we aim to support the clinical phase 2 protocol by investigating the disease impact on flucytosine (5FC) PK profile after administration of a Sustained Release (SR) formulation in the context of a switch from Immediate Release (IR) to (SR, for cryptococcal meningitis treatment in HIV patients.