About the case
- Therapeutic area: Infectious Diseases
- Development stage: Phase I
- Modeling strategy: PBPK-PBBM, MIDD (Model-Informed Drug Development)
- MIDD Impact category: Inform
Principal question
How much can the disease impact flucytosine (5FC) PK profile after administration of a SR (Sustained release) formulation in the context of a switch from Immediate Release (IR) to Sustained Release (SR), for cryptococcal meningitis treatment in HIV patients?
Impact
This work supported the clinical phase 2 protocol and the discussion with investigators (Medium impact).

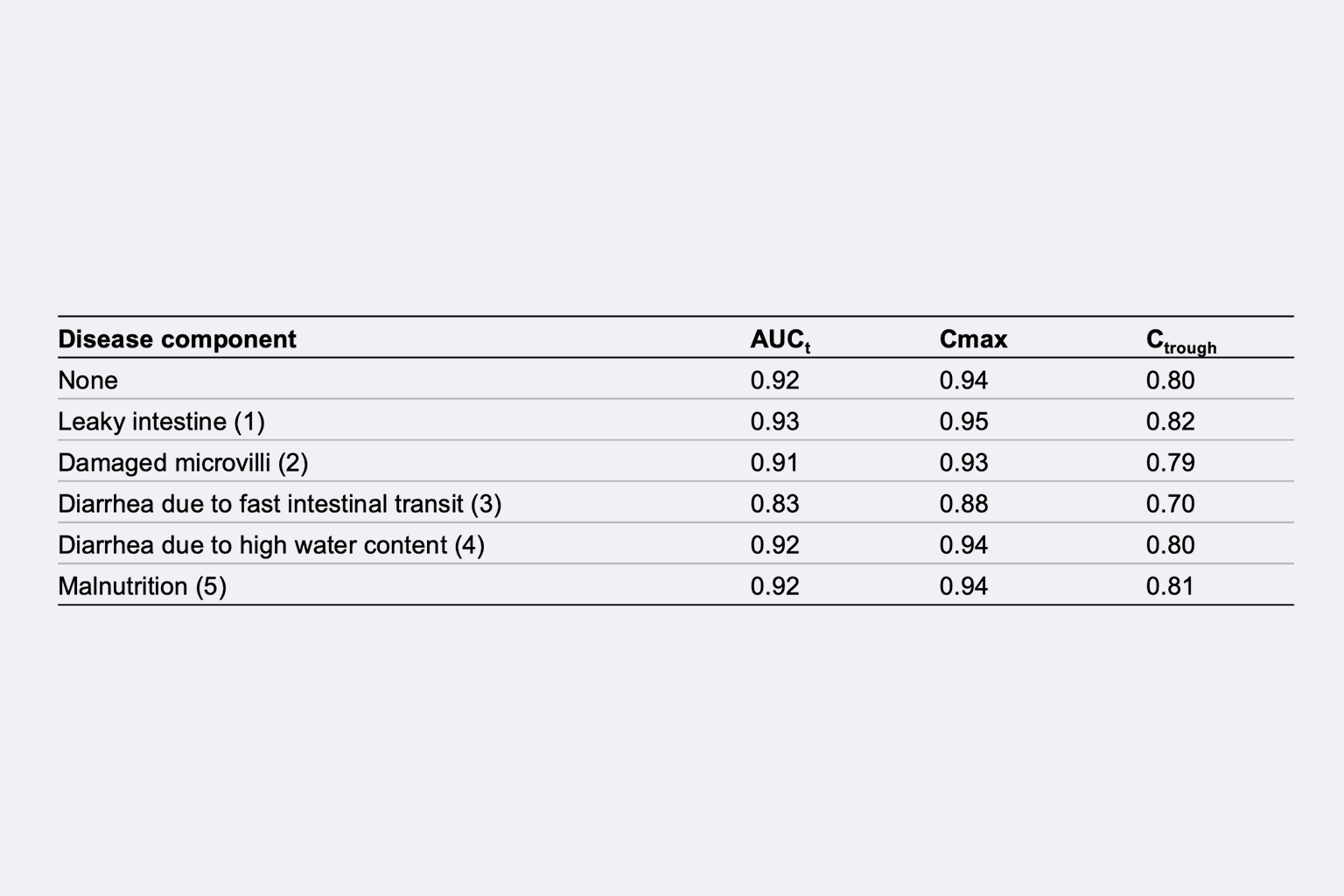
Main challenge(s)
- To predict the disease effects (i.e., leaky intestine, damaged microvilli, diarrhea due to fast intestinal transit or high-water content, and malnutrition) on 5FC PK after administration of the SR formulation based on 5FC physicochemical properties and physio-pathological knowledge.
- To generate hypotheses about disease effect on release and absorption of 5FC.
Pharmetheus role
Pharmetheus was responsible for the MIDD and the PBPK modeling and simulation, and participated in project team discussion about phase 2 protocol.
Methodological approach
Physiologically-based pharmacokinetics.
Reference
See the next case in this series
Advancing Cryptococcal Meningoencephalitis treatment with MIDD: Integrating PBPK-PBBM
Model-Informed Drug Development (MIDD) is increasingly used to improve drug formulation and dosing strategies. This case highlights how modeling and simulations played a role in supporting Drugs for Neglected Diseases initiative (DNDi).