Case overview
Utilizing Physiologically Based Pharmacokinetic (PBPK) and Physiologically Based Biopharmaceutics Modeling (PBBM) within the broader MIDD framework, our efforts contributed to informed decision-making during the development of a sustained-release formulation of flucytosine (5FC) for treating cryptococcal meningoencephalitis (CM) in HIV-infected patients.
Context
The fungus cryptococcus neoformans is often found in soil and bird dropping and people are likely to breathe in this microscopic fungus at some point in their lives, but those with advanced HIV are particularly susceptible to infection due to their weakened immune system. The fungus can then invade the lining of the brain and other organs, which quickly leads to death unless treated.
When undiagnosed and untreated, the fungal infection is fatal.
With early diagnostic and optimal treatment, the survival rate is over 70%.
With the vast majority of infections and fatalities occurring in Africa, this alarming statistic points to a critical global health disparity, highlighting how socioeconomic factors intersect with neglected diseases to create profound challenges.
Flucytosine (5FC) is a key, life-saving component of the new WHO-recommended first- and second-line treatments to CM, but access to 5FC is not guaranteed in many of the countries where the infection is a frequent serious health threat.
Where 5FC is available, it is challenging to use ― the existing formulations of this vital drug present significant hurdles in the very settings where it’s most needed. The standard 5FC dosing regimen requires administration of four oral doses ― daily ― difficult to comply with in the under-staffed and overburdened hospitals common in resource-limited environments. Furthermore, as many patients may be arriving in a state of coma, or at the very least, have problems swallowing, it is often crushed to be administered through nasogastric intubation – a pathway which the standard formulation was not designed for. This situation exemplifies how drug delivery, when not adapted to the realities, can exacerbate health inequities.
The Drugs for Neglected Diseases initiative is a not-for-profit medical research organization that discovers, develops, and delivers safe, effective, and affordable treatments for neglected people. DNDi is developing medicines for sleeping sickness, leishmaniasis, Chagas disease, river blindness, mycetoma, dengue, paediatric HIV, advanced HIV disease, cryptococcal meningitis, and hepatitis C. Its research priorities include children’s health, gender equity and gender-responsive R&D, and diseases impacted by climate change. Since its creation in 2003, DNDi has joined with public and private partners across the globe to deliver 13 new treatments, saving millions of lives.
The initiative
Recognizing these challenges, Drugs for Neglected Diseases initiative (DNDi) aimed to develop a simpler, sustained-release (SR) formulation of flucytosine (in addition to scaling up production and increasing accessibility for patients). A SR formulation is not only easier to administer – once or twice per day – but also better suited to the patients and constraints of the setting in which it is provided. By addressing these barriers, the project aims to bridge the gap between effective treatment and the socioeconomic realities faced by those most vulnerable to this deadly disease.
The planned clinical phase II trial of the the sustained release pellet form of 5FC is as of February 2025 actively enrolling participants for administration of the investigational medicinal product. The trial aims to evaluate the pharmacokinetics, safety, tolerability, and preliminary efficacy of this new formulation and marks a milestone of progress – significantly accelerated and made possible by a robust Model-Informed Drug Development (MIDD) strategy, specifically the application of Physiologically Based Pharmacokinetic (PBPK) modeling.
Case study
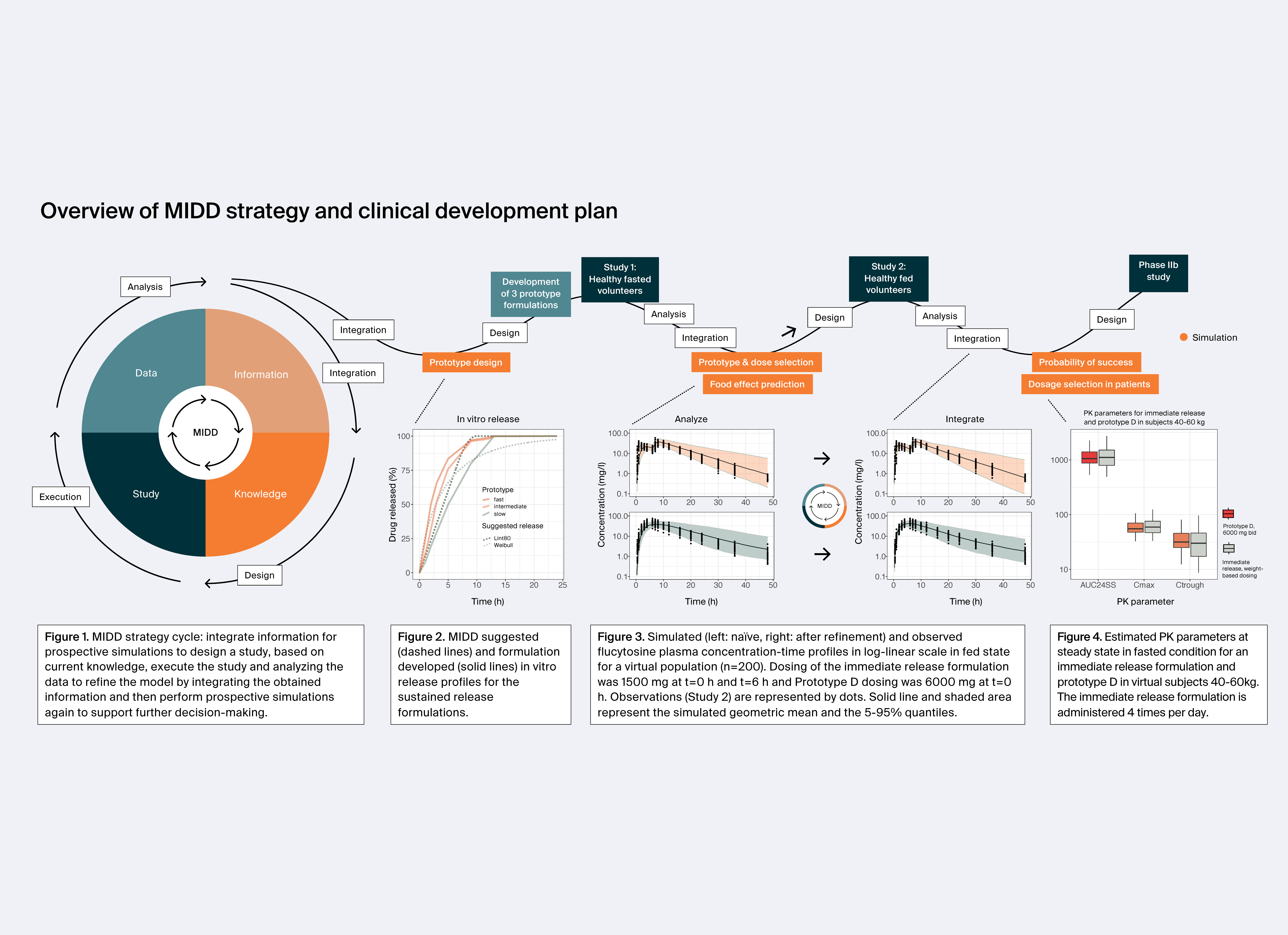
MIDD strategy and execution
Early model integration and iterative refinements, a core component of MIDD, facilitated informed decision-making throughout the drug development process.
Objective
The primary goal of this study was to demonstrate the value of MIDD and PBPK modeling in the development of an SR formulation of flucytosine. The research aimed to optimize dosing strategies and improve drug exposure in patients with cryptococcal meningoencephalitis.
Methodology
PBPK model development: An initial PBPK model was created based on limited literature data.
Refinement & validation: The model was refined iteratively using data from two Phase 1 clinical studies with different flucytosine formulations under various prandial conditions in healthy participants.
SR formulation design: The model was applied to guide SR prototype formulation development and dose selection.
Clinical application: The PBPK model was used to determine dosage for an upcoming Phase 2 clinical study, with a focus on low-weight patients.
Ad hoc simulations: The flexibility of MIDD allowed for quick assessments of ancillary questions, such as:
- Adding a loading dose for SR treatment.
- Assessing drug exposure in unconscious patients to refine therapeutic strategies.
Key findings
- Optimized dose selection: The PBPK model helped determine appropriate SR formulation doses for different patient populations, particularly low-weight individuals.
- Rapid decision-making: MIDD enabled fast and effective assessments of additional strategies to enhance treatment efficacy.
- Improved therapeutic outcomes: Model integration throughout the drug development process ensured adaptive, reliable decision-making, leading to a well-optimized SR flucytosine formulation.
Conclusion
This case study highlights the transformative potential of MIDD in accelerating drug development. PBPK modeling played a crucial role in optimizing the design and dosing of the SR formulation of flucytosine for CM treatment. The continuous integration of knowledge ensured model adaptability, demonstrating the effectiveness of MIDD in addressing evolving clinical challenges and improving patient outcomes.
Connected case studies
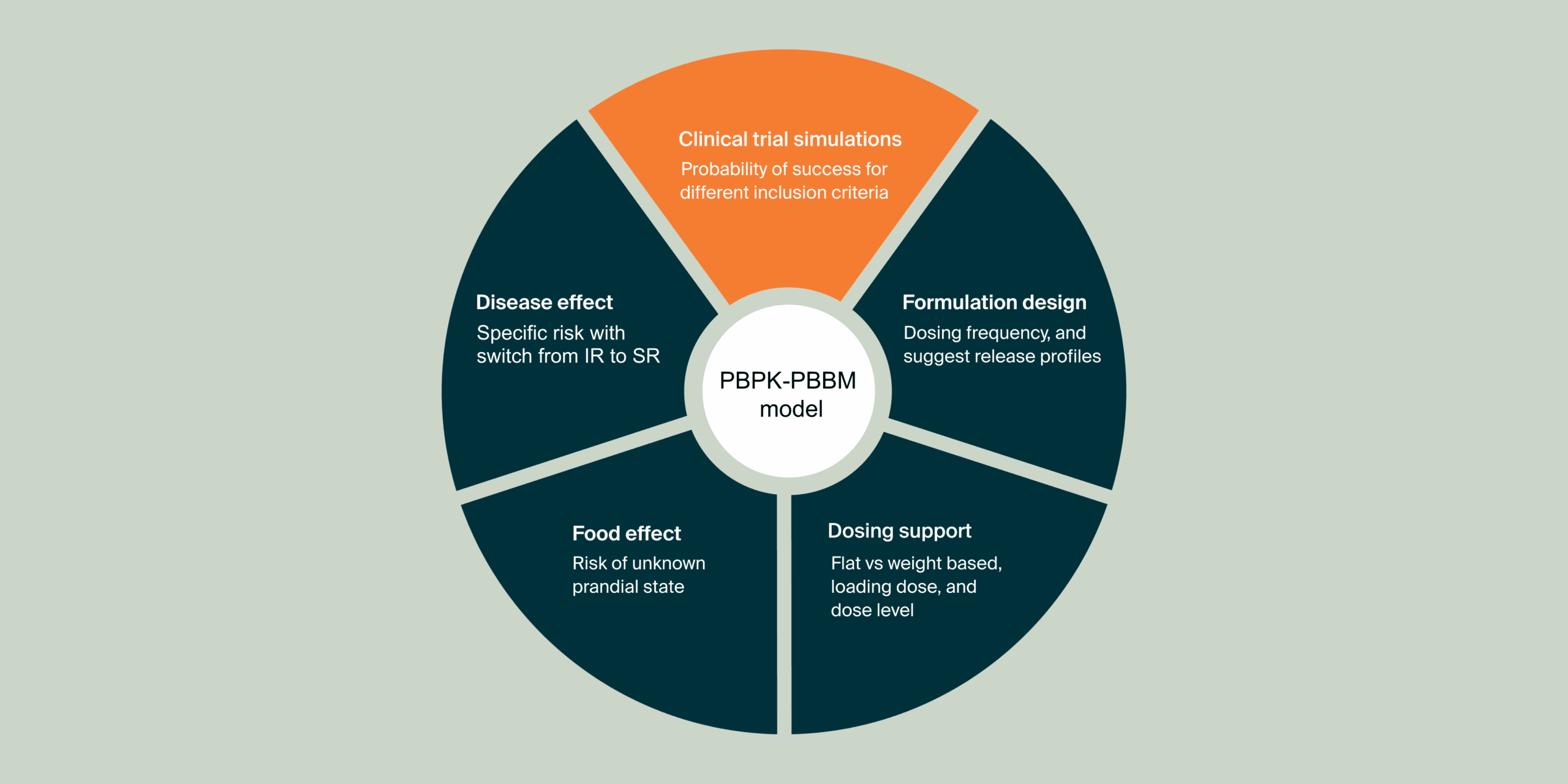
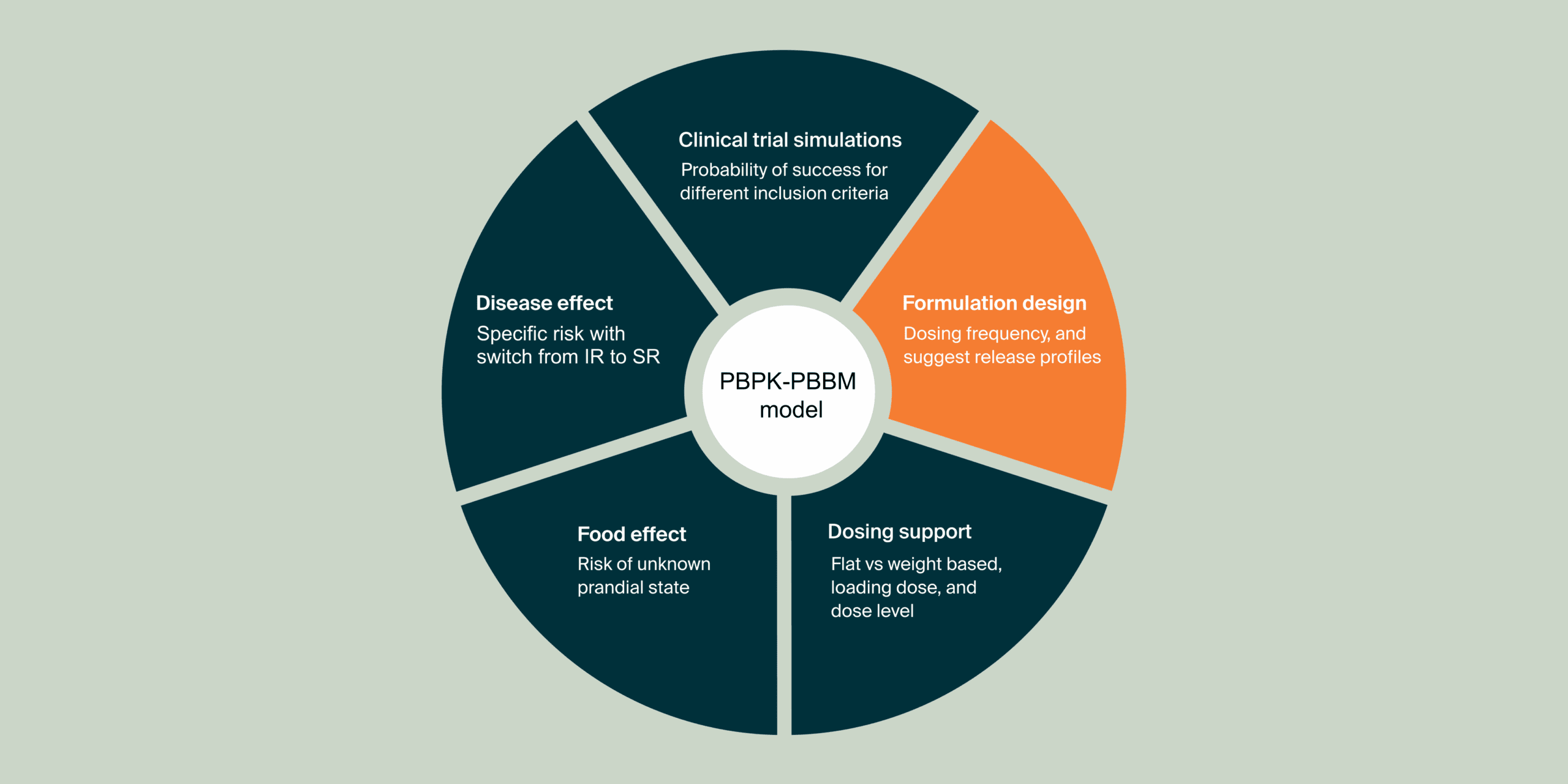
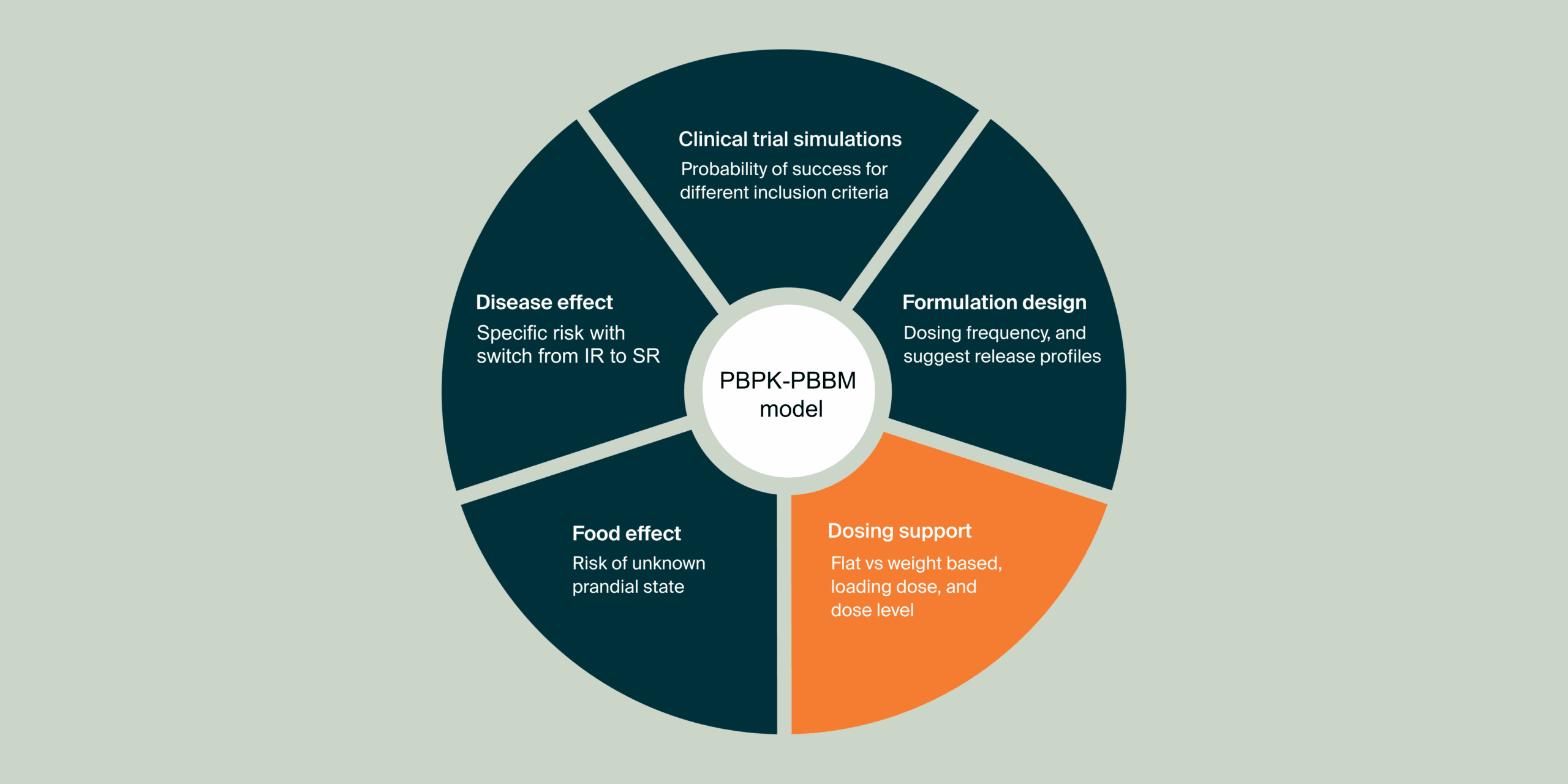
These additional case studies highlight individual components of the systematic MIDD approach. They centrally focus on the application of physiologically-based pharmacokinetic (PBPK) modeling and simulation to understand and predict how various formulations influence drug behavior within the body.
The first study focuses on the PBPK-informed design of sustained-release (SR) formulation prototypes. It aimed to determine the in vitro dissolution profile necessary for an SR formulation to meet target pharmacokinetic (PK) profiles with twice-daily administration. This study relied on PBPK models developed primarily from literature data.
The second study built upon the first by focusing on PBPK-informed selection of a specific SR formulation prototype and its appropriate dosage for further clinical evaluation. This stage incorporated initial clinical data to refine the models and support decisions for subsequent trials, with findings presented to the WHO.
The third study investigated the food effect on the pharmacokinetic (PK) profile of the selected SR formulation when transitioning from an immediate-release (IR) formulation. This analysis aimed to predict how food intake might influence drug absorption and exposure, ultimately informing recommendations for patients regarding food intake.
The fourth study investigated how the disease can impact flucytosine (5FC) PK profile after administration of a Sustained Release (SR) formulation in the context of a switch from an Immediate Release (IR) to a SR formulation for cryptococcal meningitis treatment in HIV patients. The results from this study supported the clinical phase 2 protocol and the discussion with investigators.
Collectively, these excerpts illustrate a comprehensive MIDD strategy which integrate PBPK and PBBM modeling to guide the development of sustained-release formulations of flucytosine. The overarching objective is to achieve target drug concentrations with potentially less frequent dosing, ultimately aiming to improve patient outcomes in the treatment of cryptococcal meningitis in HIV-positive individuals
Connected publications
Explore these publications focusing on specific parts of this project
Related posters & presentations
Interested in the latest advancements in drug development?
Explore our research, subscribe for updates, or contact us for collaboration opportunities.
Discover more about PBPK-PBBM modeling →
Learn more about MIDD →
Subscribe to our newsletter →
Contact us →