About the case
- Therapeutic area: Infectious Diseases
- Development stage: Phase I
- Modeling strategy: PBPK-PBBM, MIDD (Model-Informed Drug Development)
- MIDD Impact category: Inform
Principal question
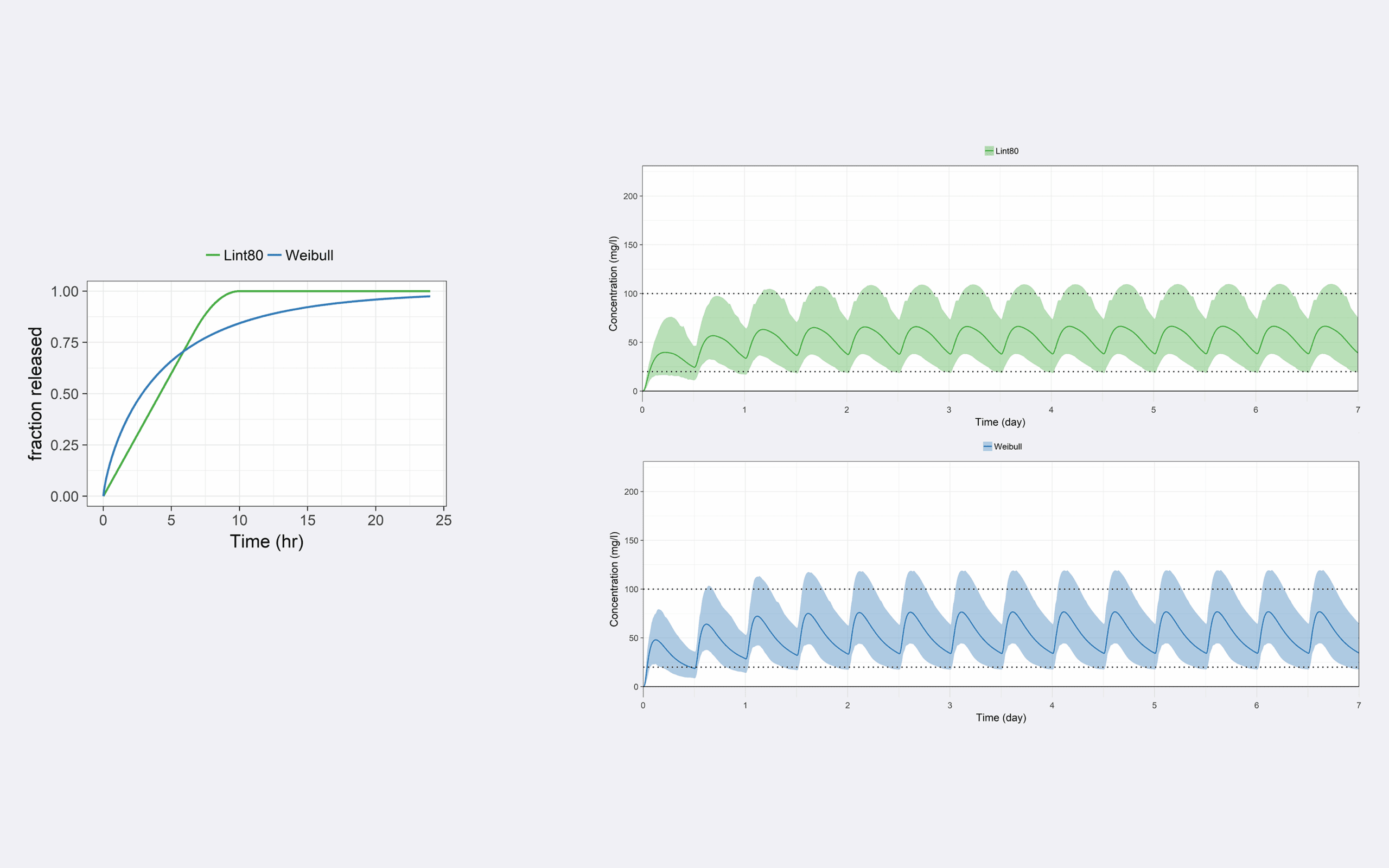
What is the in vitro dissolution profile of a sustained release prototype formulation of flucytosine (5FC) allowing to meet target PK profiles, with a twice daily administration, in HIV patients with cryptococcal meningitis?
Impact
This work supported the design of the 3 sustained release prototypes for a clinical study aiming at comparing 3 different release rate (slow, intermediate and fast) sustained release formulations of 5FC to the existing immediate release formulation (Medium impact).
Main challenge(s)
PBPK model developed solely from literature data (no IV data, only per os).
Pharmetheus role
Pharmetheus was responsible for the MIDD strategy and the PBPK modeling and simulation. Pharmetheus proposed 2 different in vitro release rate profiles which should allow to meet PK target profile and lead to sufficiently different in vivo PK profiles (20% difference).
Methodological approach
Physiologically-based biopharmaceutics modelling (PBBM), physiological in vitro in vivo relationship (IVIVR) by convolution.
See the next case studie in this series
PBPK-informed selection of formulation prototype and dosage in the context of a sustained release formulation development
In this PBPK-informed case study, we support the selection of a sustained release (SR) formulation prototype and optimal dosage for flucytosine (5FC) in HIV patients with cryptococcal meningitis by refining the legacy model , solely built with literature data, in integrating the first clinical PK data with the SR formulation prototypes of 5-FC.