PBPK-PBBM consulting services
Physiologically-based pharmacokinetics (PBPK) and physiologically-based biopharmaceutics modeling (PBBM) leverage accumulated knowledge to inform mechanistic analyses and achieve predictions beyond direct observations. These models integrate physiological and drug-specific parameters to optimize drug development, refine dosing strategies, and enhance risk assessment.

Benefits
Allow for scenarios beyond those observed
The independent representations of drug, biological entities and system parameters in PBPK-PBBM models allow for extrapolation to scenarios beyond those observed. We see the main benefits with applying PBPK-PBBM analyses as the following:
- Distinctively addresses how a drug is absorbed, distributed, and eliminated, which allows for better understanding of each process and their respective association to drugs exposure and dynamics.
- Via biopharmaceutics and administration route specific absorption models provide basis for optimized drug delivery, drug formulation and dosing strategies to improve drug performance and decisions in bridging.
- PBPK facilitates predictions of drug concentration at the site of pharmacological action. Combined with a model representing pharmacological response this provides a holistic and mechanistic understanding of the concentration/response relationship(s) of drugs in patients.
- Common translational applications for PBPK-PBBM modeling are First-In-Human trials support, biopharmaceutics and virtual bioequivalence (VBE) assessments, drug-drug interactions (DDI), special populations, like pediatrics and organ ,, or early prediction of clinical efficacy.
Way of working
At Pharmetheus, we always make an assessment from the perspectives of necessity and possibility in the design of an efficient strategy to address your current needs. This to determine how to best leverage current knowledge to reach set objectives, while keeping an eye open to future potential applications. Projects are conducted according to Pharmetheus’ way of working to ensure reproducibility and quality in deliverables of regulatory standards. Our clients have submitted reports to the authorities with our support.
Tools
Physiologically-based pharmacokinetics/physiologically-based biopharmaceutics modeling (PBPK-PBBM) is often associated with an expensive and closed licensed tool.
In-depth examples
Pharmetheus leads its own research initiatives and collaborates with a range of subject matter experts. Below you can read more about one of those initiatives.
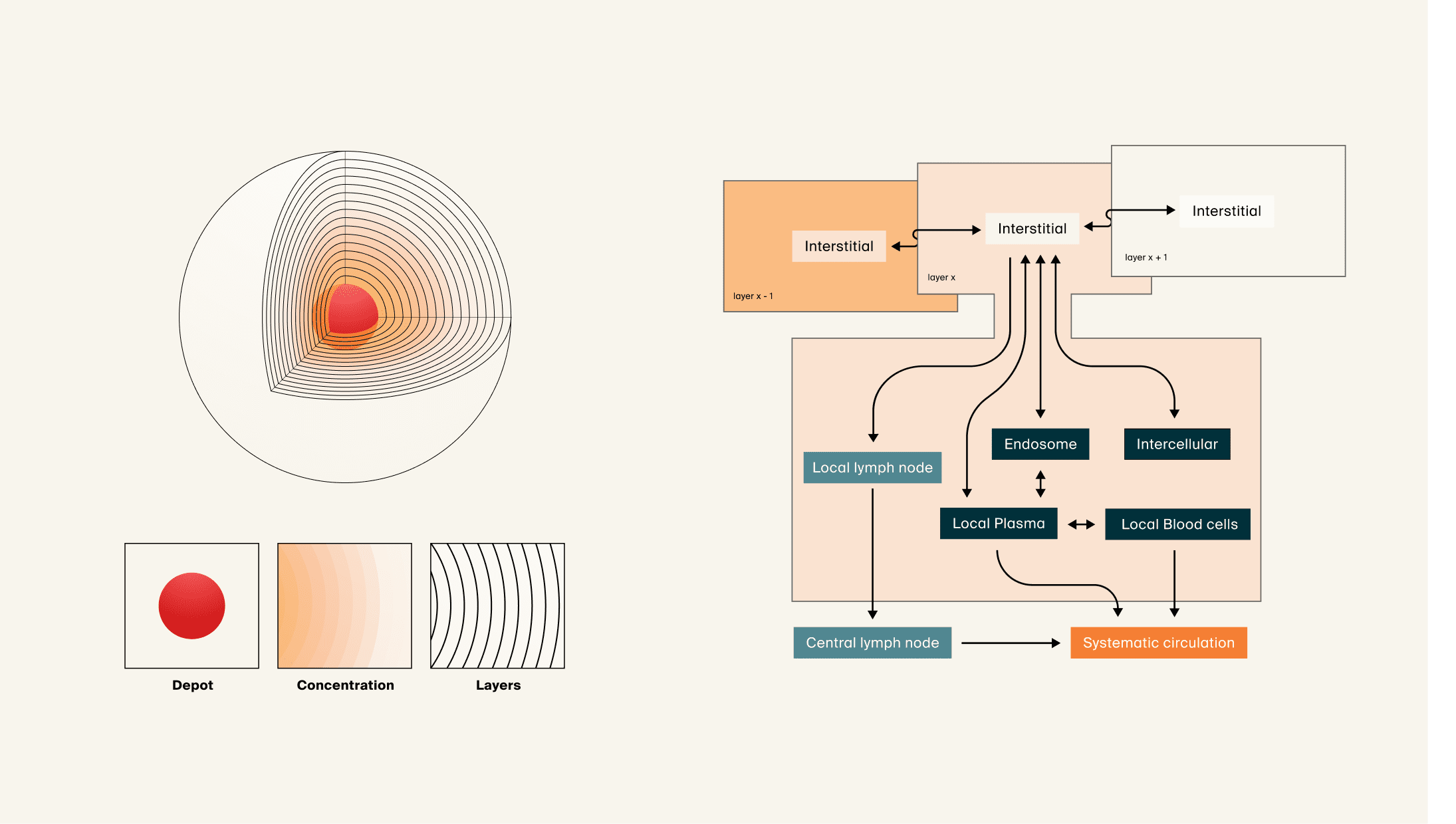
Subcutaneous drug delivery: PBPK-QSP model for absorption and anti-drug antibody response
Get in touch

How can we help you?
Contact us to discuss how we can help you, or request a proposal
Featured afilliated publications
Explore our collaboration forms
At Pharmetheus, we understand that each organization has unique challenges and objectives in drug development. That’s why we offer a range of collaboration models designed to provide flexible, high-impact support tailored to your specific needs. Whether you require comprehensive PBPK-PBBM solutions, targeted scientific insights, or hands-on training, our expert team is ready to collaborate in a way that best suits your project.