
Importance of regulatory strategy
Dr. Tomas Salmonson, a Pharmetheus board member who has chaired the Committee for Medicinal Products for Human Use, CHMP, at the European Medicines Agency (EMA), comments:
– Regulatory strategy is becoming increasingly crucial to bring new drugs to market. The main responsibilities of this role are to monitor and report regulatory news, analyze changes and recommend actions, and to enable tailored training, both internally within the company and to external audiences.
Multifaceted perspective
Dr. Emma Hansson is starting out in the role with a clear direction:
– Re-designing a study is costly and adds on time-to-market. The complexity of the regulatory landscape can be hard to navigate. Pharmetheus intensifying competencies in this area is important as the future of drug development will be even more demanding. This way we ensure our clients keep getting the best regulatory strategy support, says Dr. Emma Hansson.
The appointment of Emma Hansson is adding to the company’s capabilities within the field. Emma joined Pharmetheus in 2016, from the Medical Product Agency (MPA) in Sweden where she worked as a Pharmacokinetics and Pharmacometrics Assessor. Her experiences gained at MPA, and from being a member of the expert group on Modeling and Simulation at the European Medical Agency, (EMA), and from her work as an MIDD consultant provide her with a unique combination of skills and expertise, directly benefiting the clients:
– I have a solid understanding and insights to the assessment of applications of new drugs. Having worked as an MIDD consultant in numerous drug development projects, and at regulatory agencies, I can bring a multifaceted perspective to the table, says Emma Hansson.
Increasing the regulatory affairs focus in the drug development process can decrease both time to market and cost. Advising clients in the study design phase on regulatory strategy or how to best use modeling and simulation as supporting evidence to comply with the latest regulatory requirements is highly valuable.
MIDD Adoption progress
– Agencies globally are increasingly engaging with MIDD and advancing the use of it. To me, to Pharmetheus and its clients, not to forget patients, it’s promising. It is through regulatory guidance and pilot programs the application of MIDD will become best practice in drug development, says Tomas Salmonson.
Pharmetheus’ existing services within regulatory are several; from expert advice and regulatory indication services, to strategies within MIDD as well as continuous advising throughout projects. What this role will do is take the regulatory strategy framework to the next level.
– This new role is really exciting and I am looking forward to further developing Pharmetheus regulatory strategy services, says Emma Hansson.
Regulatory Support
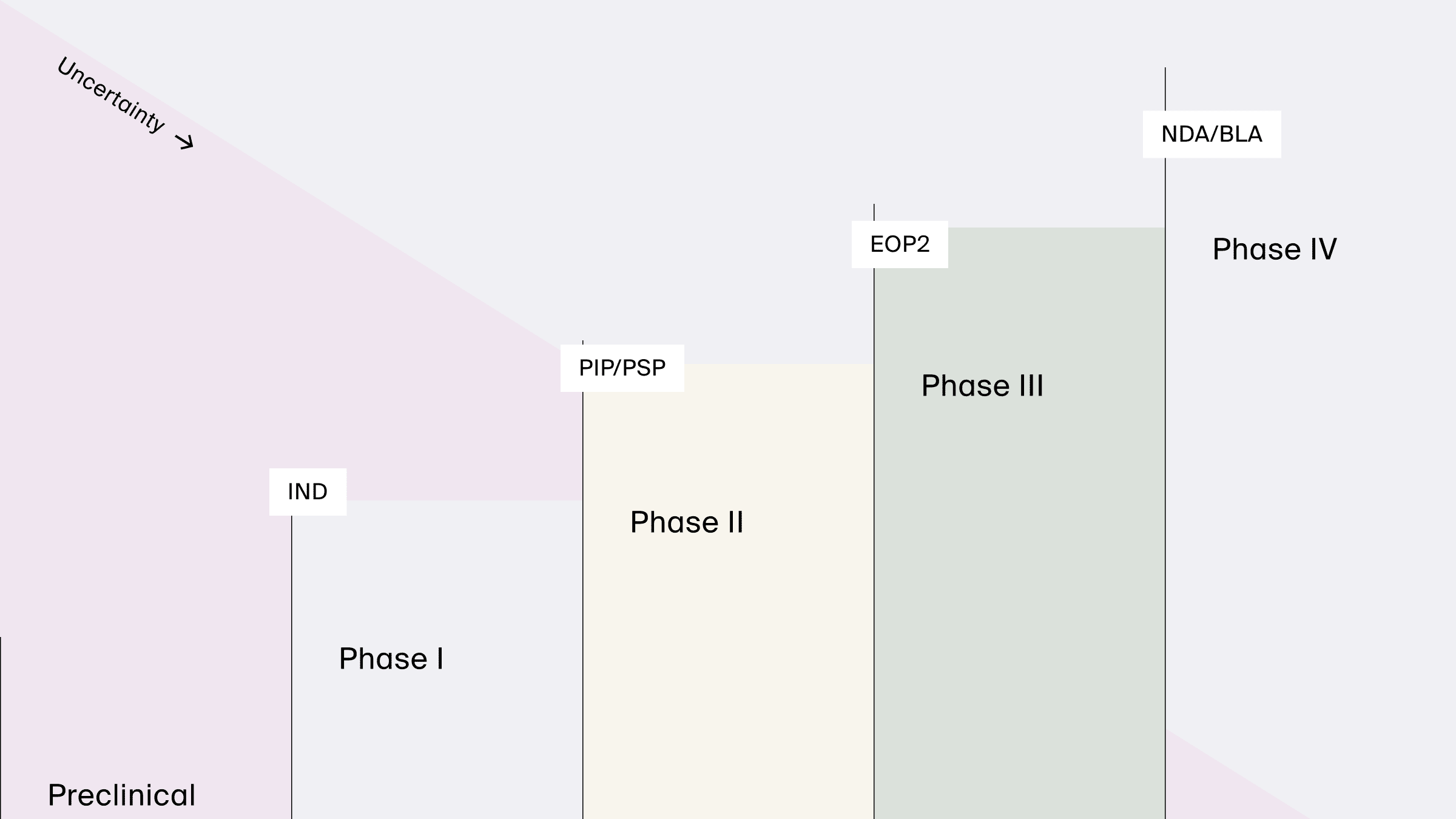
Regulatory interactions
Reduction in uncertainty obtained as Phases of drug development progress and modeling supports key regulatory interactions, from pediatric plans (PIP/PSP) to new drug or biologics license applications (NDA/BLA).
