About the case
- Therapeutic area: Infectious disease
- Phase IV
- Impact of Model Informed Drug development: Informing
Conclusion
The deliverable was used to support Go-No Go decision for the development of MAP with anti-retroviral drugs to treat children with HIV and was submitted to NIH. PATH has obtained a 3-years founding from NIH to pursue this work.
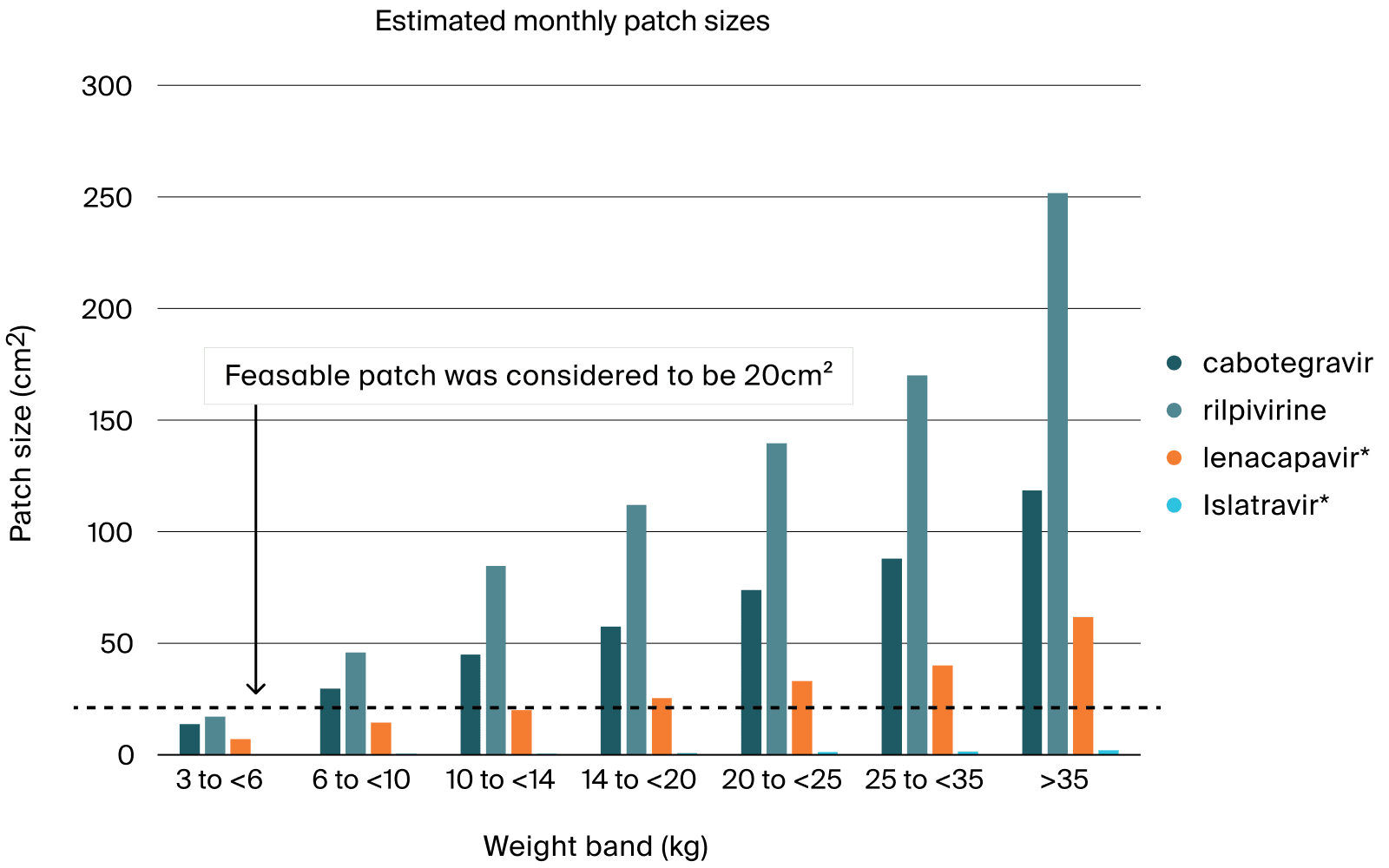
- Main challenges were the initial lack of information of the Map release performance, the complex pharmacokinetics properties of the pre-selected anti-retroviral drugs, and the challenging timelines driven by the interaction with NIH.
- Pharmetheus performed hands-on modelling and prepared the deliverable to be shared with NIH.
- Methodologically, a physiologically based pharmacokinetics (PBPK) approach was chosen for this analysis to handle the complex pharmacokinetics of anti-retroviral drugs, the need of age-dependent scaling of ontogeny, and the physiology and scaling of the site of application.